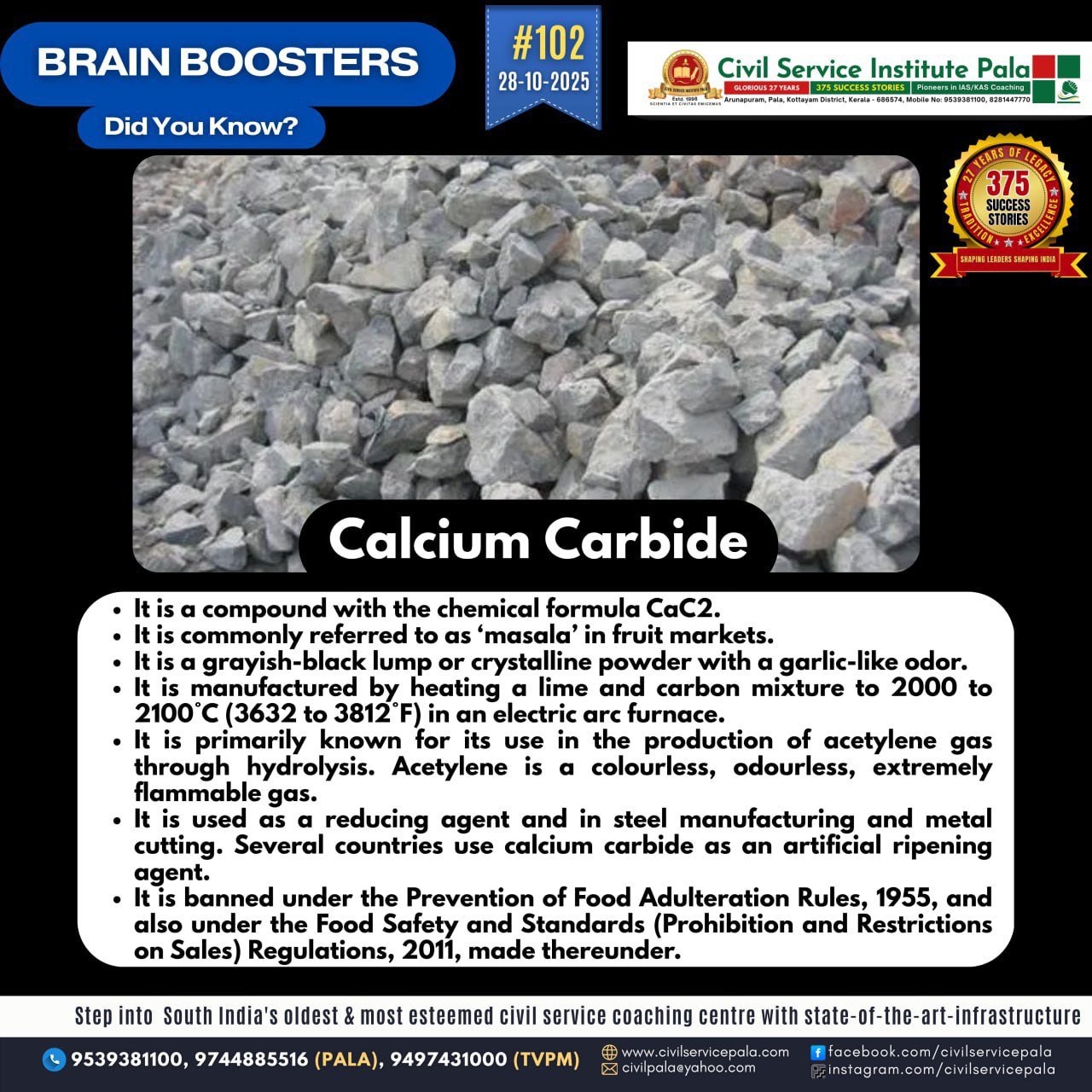
Calcium Carbide
Chemical formula: CaC₂ Appearance: Greyish-white solid; emits a strong garlic-like odor when wet.
Composition: Made up of calcium (Ca) and carbon (C₂).
Reaction with water: CaC₂ + 2H₂O → C₂H₂ (acetylene gas) + Ca(OH)₂ CaC₂ + 2H₂O → C₂H₂ (acetylene gas) + Ca(OH)₂ → This reaction produces acetylene gas, which is used for welding and lighting. Uses Production of acetylene gas – for metal cutting, welding, and as a raw material in chemical industries. Manufacture of calcium cyanamide – used as a fertilizer and pesticide. Used in steelmaking – acts as a deoxidizer and desulfurizer. Fruit ripening agent – (illegally used in some places) to ripen fruits like mangoes and bananas. Hazards Contact with water releases flammable acetylene gas, which can cause explosions. Health risk: The gas and impurities like arsenic and phosphorus can be toxic. Not safe for use in food ripening due to harmful residues.
